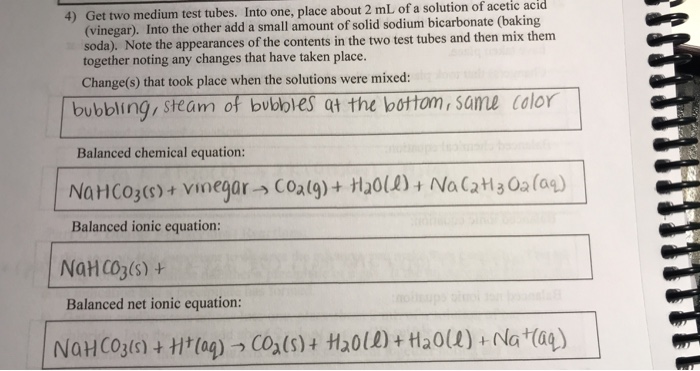Notice: Trying to access array offset on value of type null in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 76
Warning: shuffle() expects parameter 1 to be array, null given in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 78
The product of vinegar CH3COOH and baking soda NaHCO3 is water carbon dioxide gas and aqueous sodium acetate CH3COONa. We will use a weak acetic acid solution vinegar about 4 to 5 acetic acid CH3COOH in water mixed with baking soda which is nearly 100 sodium bicarbonate NaHCO3. Component of baking soda. The complete reaction would be NaHCO3 HC2H3O2 Na. B Based on your observations explain the entropy change for the system observed in Step 1. 49A Natural AcidBase Indicators. Baking soda sodium bicarbonate plus vinegar acetic acid yields carbon dioxide plus water plus sodium ion plus acetate ion. D Write the overall equation and the net ionic equation for the process. Its chemical formula is NaHCO 3 meaning its made of one sodium atom one hydrogen atom one carbon atom and three oxygen atomsVinegar is a mixture of acetic acid and water. Vinegar contains acetic acid and baking soda contains sodium hydrogen carbonate.
Notice: Trying to access array offset on value of type null in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 76
Warning: shuffle() expects parameter 1 to be array, null given in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 78
49A Natural AcidBase Indicators. The equilibrium shifts tow. Red Cabbage Juice Extract. Dehydrated red cabbage juice Scoop and plastic beaker. C Use the entropy data from Table 1 to calculate the entropy change for the. The chemical name for baking soda is sodium bicarbonate. Chemical Equation Baking Soda And Vinegar. This reaction is often used in volcano demonstrations. As an example lets use the reaction of baking soda sodium bicarbonate and vinegar acetic acid and lets have them both be aqueous and assume only dissociated ions of acetic acid react. D Write the overall equation and the net ionic equation for the process.
Notice: Trying to access array offset on value of type null in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 76
Warning: shuffle() expects parameter 1 to be array, null given in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 78
The product of vinegar CH3COOH and baking soda NaHCO3 is water carbon dioxide gas and aqueous sodium acetate CH3COONa. Tuesday April 16 2019. Chemical reactions - AcidsBases Description. Step 2 a Define entropy and the significance of the sign of its value. What is the net ionic reaction when vinegar and baking soda react. Vinegar contains acetic acid and baking soda contains sodium hydrogen carbonate. The relative pH of some foods and household chemicals is illustrated using red cabbage juice indicator. The reaction between baking soda sodium bicarbonate and vinegar acetic acid. This chemistry video tutorial discusses the reaction between baking soda and vinegar. Write an equation with phases for the reaction between nitric acid and calcium carbonate Write the net ionic equation with phases for this reaction Determining the Concentration as a Volume Percentage Trial 3 Volume of vinegar ml Moles of acetic acid reacted Trial 1 Trial 2 loonL 75ml 125ml 01261 01130 O1619 7579 6759 9729 721 mL 6.
Notice: Trying to access array offset on value of type null in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 76
Warning: shuffle() expects parameter 1 to be array, null given in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 78
BAKING SODA AND VINEGAR. D Write the overall equation and the net ionic equation for the process. The chemical equation for the overall reaction is. _____ Net ionic equation. We will use a weak acetic acid solution vinegar about 4 to 5 acetic acid CH3COOH in water mixed with baking soda which is nearly 100 sodium bicarbonate NaHCO3. 3 NaHCO3 s CH3COOH l H2O l CO2 g CH3COONa aq. C Use the entropy data from Table 1 to calculate the entropy change for the. Chemical reactions - AcidsBases Description. 49A Natural AcidBase Indicators. Baking soda sodium bicarbonate plus vinegar acetic acid yields carbon dioxide plus water plus sodium ion plus acetate ion.
Notice: Trying to access array offset on value of type null in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 76
Warning: shuffle() expects parameter 1 to be array, null given in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 78
C Use the entropy data from Table 1 to calculate the entropy change for the. _____ Classify the type of reaction that occurred in each case or types as a reaction may fall under more than one category. The reaction between baking soda and vinegar actually occurs in two steps but the overall process can be summarized by the following word equation. Step 2 a Define entropy and the significance of the sign of its value. One example is the reaction between vinegar and baking soda. With s solid l. As an example lets use the reaction of baking soda sodium bicarbonate and vinegar acetic acid and lets have them both be aqueous and assume only dissociated ions of acetic acid react. Net ionic equation Here we cancel the ions that appear on each side of the equation. Write the complete and net ionic equations with phases for the reaction that occurred placing a piece of MAGNESIUM IN VINAGER. This chemistry video tutorial discusses the reaction between baking soda and vinegar.