Notice: Trying to access array offset on value of type null in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 76
Warning: shuffle() expects parameter 1 to be array, null given in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 78
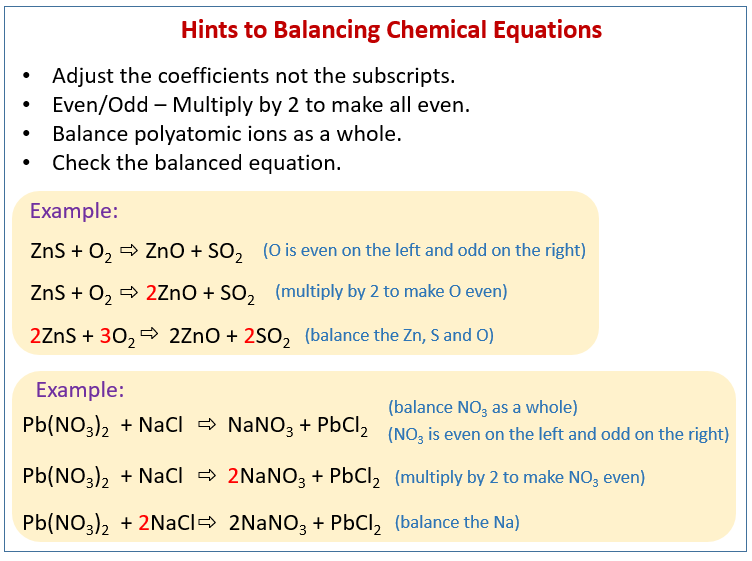
What is the example of unbalanced chemical equation. This collection of ten chemistry test questions will give you practice in how to balance chemical reactions. Balancing chemical equations is a basic skill in chemistry and testing yourself helps retain important information. In the reaction Mg O2 MgO the number of atoms of each element on either side of the arrow is not equal. Chemical reactions have the same number of atoms before the reaction as after the reaction. The unbalanced chemical equation must be obtained by writing the chemical formulae of the reactants and the products. Balancing Chemical Equations. Balancing Equations Difficult Ones In AQA exams there are commonly some quite difficult balancing equations questions. H 3 PO 4 NaOH Na 3 PO 4 H 2 O. 2Al 6HCl 2AlCl 3 3H 2.
Notice: Trying to access array offset on value of type null in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 76
Warning: shuffle() expects parameter 1 to be array, null given in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 78
This is a set of worked examples of how to balance chemical equations a very important skill in chemistry. Example of balancing chemical equation by the algebraic method. In this example it can be difficult to know where best to start as 3 of the elements appear once in the reactants and products. FeCl3 3NH4OH --- FeOH3 3NH4Cl And if you count up the atoms on each side you will see that this is now. The values of all the algebraic variables are substituted into the chemical equation to obtain the balanced chemical equation. Al 2 SO 4 3 Ca OH 2 Al OH 3 CaSO 4. Take for example the last chemical equation which we balanced. The next most obvious unbalanced part is that there are now three NH4groups on the right but only one on the left hand side. Write Down the Unbalanced Equation. FeCl 3 MgO --- Fe 2 O 3 MgCl 2.
Notice: Trying to access array offset on value of type null in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 76
Warning: shuffle() expects parameter 1 to be array, null given in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 78
Balancing chemical equations is a basic skill in chemistry and testing yourself helps retain important information. Sixteen balance redox equations by sight. So with one final adjustment we get our final answer. In this example it can be difficult to know where best to start as 3 of the elements appear once in the reactants and products. Such an equation which has an unequal number of atoms of one or more elements in reactants and products is called unbalanced chemical equation. Note that if you have 1 of something it does not get a coefficient or subscript. Example 4 Unbalanced equation- Na2CO3 HCl --- NaCl CO2 H2O Well the sodium Na is not balanced yet and is in one chemical species on each side so we need two NaCl on the right agreed. Converse to equation 1 in equation 2 and 3 number of atoms of various elements present in reactants and products are not same. Chemical reactions have the same number of atoms before the reaction as after the reaction. FeCl 3 MgO --- Fe 2 O 3 MgCl 2.
Notice: Trying to access array offset on value of type null in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 76
Warning: shuffle() expects parameter 1 to be array, null given in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 78
Balancing Equations Difficult Ones In AQA exams there are commonly some quite difficult balancing equations questions. Easy Equation Balancing Examples. FeCl 3 MgO --- Fe 2 O 3 MgCl 2. This collection of ten chemistry test questions will give you practice in how to balance chemical reactions. Therefore this is an unbalanced chemical equation. Balance each of the following equations. C5H12 O2 CO2 H2O. Balancing chemical equations examples questions and answers. Balance the following chemical equations. Twenty examples Problems 11-25 Problems 26-45 Problems 46-65.
Notice: Trying to access array offset on value of type null in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 76
Warning: shuffle() expects parameter 1 to be array, null given in D:\laragon\www\shurikenbuataddsite\cache\5c1f467b453975b58afcbd00ae09512d2878f200.php on line 78
The first step to balance the equation is to write down the chemical formula of reactants that are listed on the left side of the chemical equation. This is a set of worked examples of how to balance chemical equations a very important skill in chemistry. Twenty examples Problems 11-25 Problems 26-45 Problems 46-65. Six balancing by groups problems Only the problems Return to Equations Menu. But if you notice on the product side element lacks any subscript. Example of balancing chemical equation by the algebraic method. Balancing chemical equations is a basic skill in chemistry and testing yourself helps retain important information. These equations can have multiple solutions and the minimum values of the variables are to be selected. Easy Equation Balancing Examples. Balanced or easy-to-balance chemical equations will be seen in the following examples.